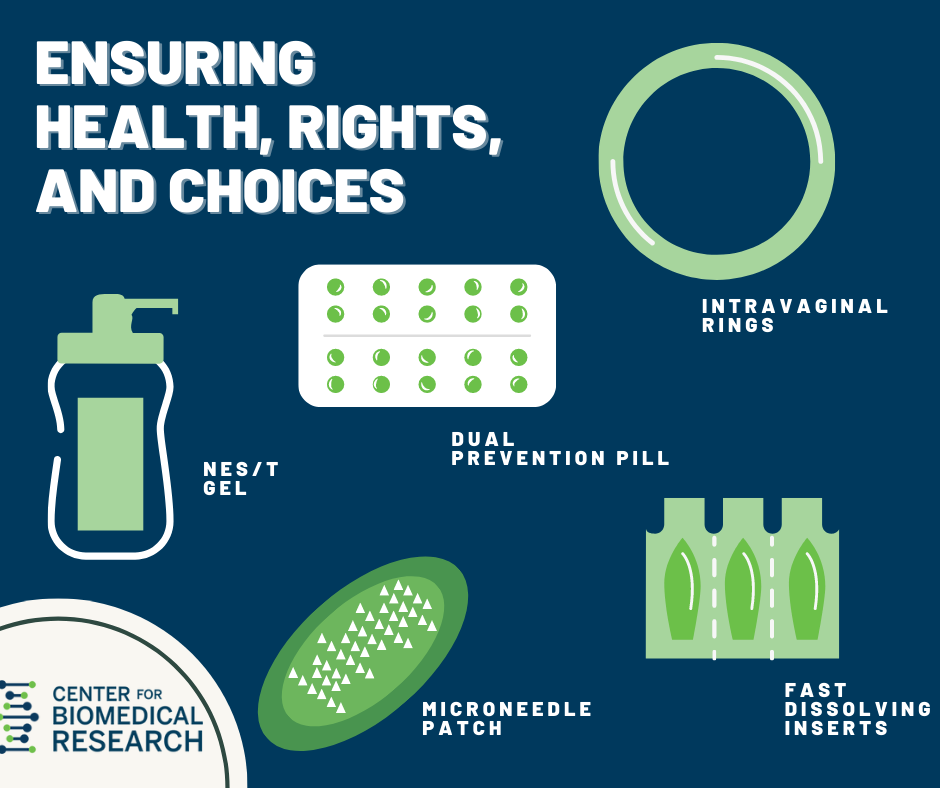
From IUDs to implants and vaginal rings, our Center for Biomedical Research (CBR) has brought nine products to market. More than 170 million women around the world use contraceptives developed by CBR or based on our technologies.
Still, 200 million women worldwide have an unmet need for a modern method of contraception; 1.5 million people are newly infected with HIV every year; and more than 1 million people acquire a sexually transmitted infection every day.
A range of product options is essential to tackle the ongoing burdens of unintended pregnancy, HIV, and sexually transmitted infections. CBR’s innovations offer individuals with options that meet their needs and protect sexual and reproductive health at various points in their lives.
This World Contraception Day, we spotlight the next generation of safe, effective contraception and HIV and STI prevention options that CBR scientists are developing to ensure equity and choice.
Nestorone®/Testosterone Transdermal Gel for Male Contraception
Men need more ways than just condoms and vasectomies to help shoulder the burden of avoiding unintended pregnancy. In collaboration with the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), we are testing a reversible, easy-to-use contraceptive gel for men.
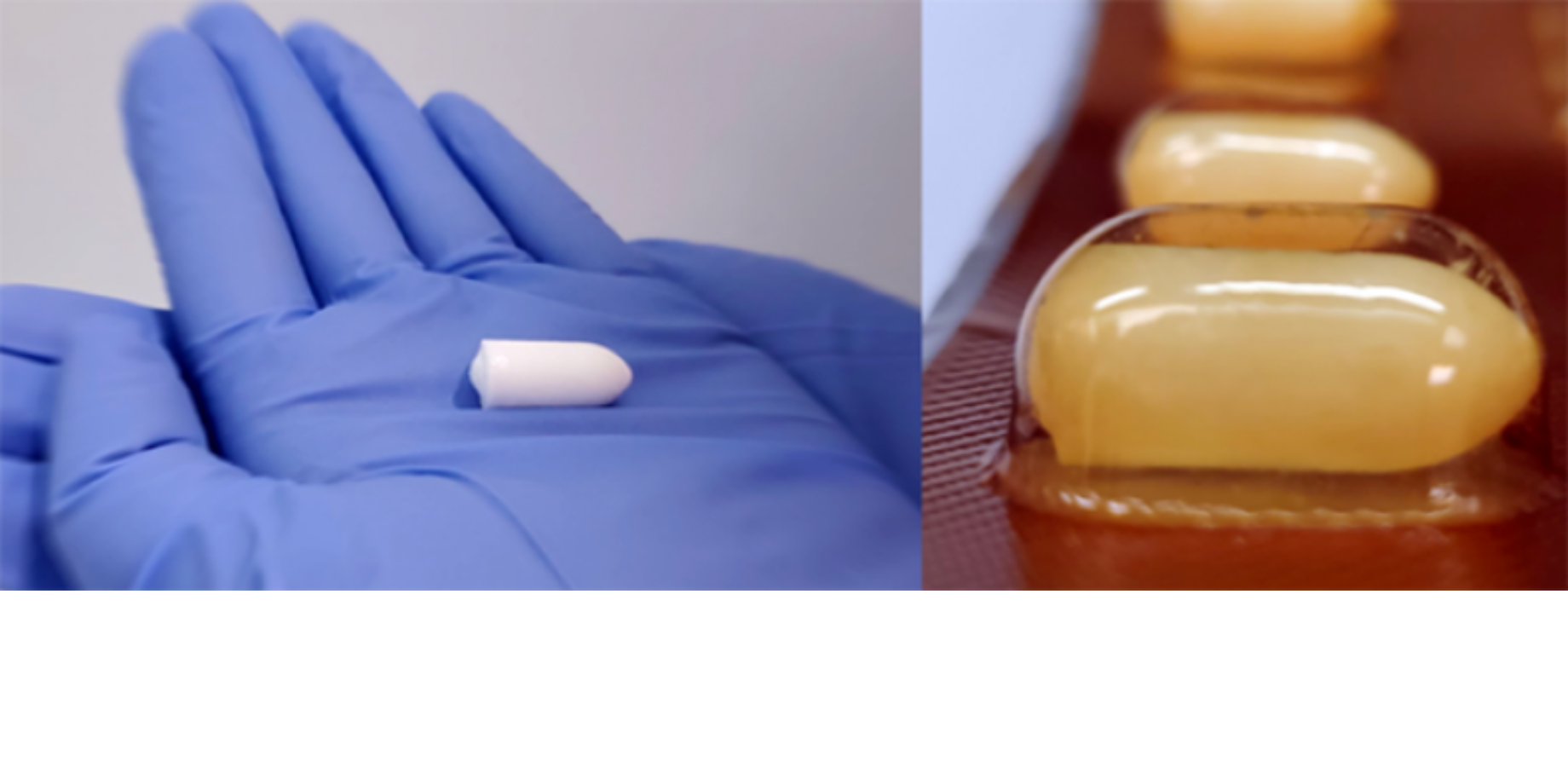
Microneedle Patch for Discreet, On-Demand Contraception
Together with GeorgiaTech, we’re developing a small, removable patch that can deliver a low dose of hormones for discreet, on-demand contraception when applied prior to sex.
Non-hormonal Contraceptive Multipurpose Prevention Technology (MPT) Containing Q-Griffithsin
Over 1 million people worldwide contract an STI every day. If effective, our nonhormonal, user-controlled, fast-dissolving vaginal insert could simultaneously protect against HIV and several other STIs, unintended pregnancy, and bacterial vaginosis.
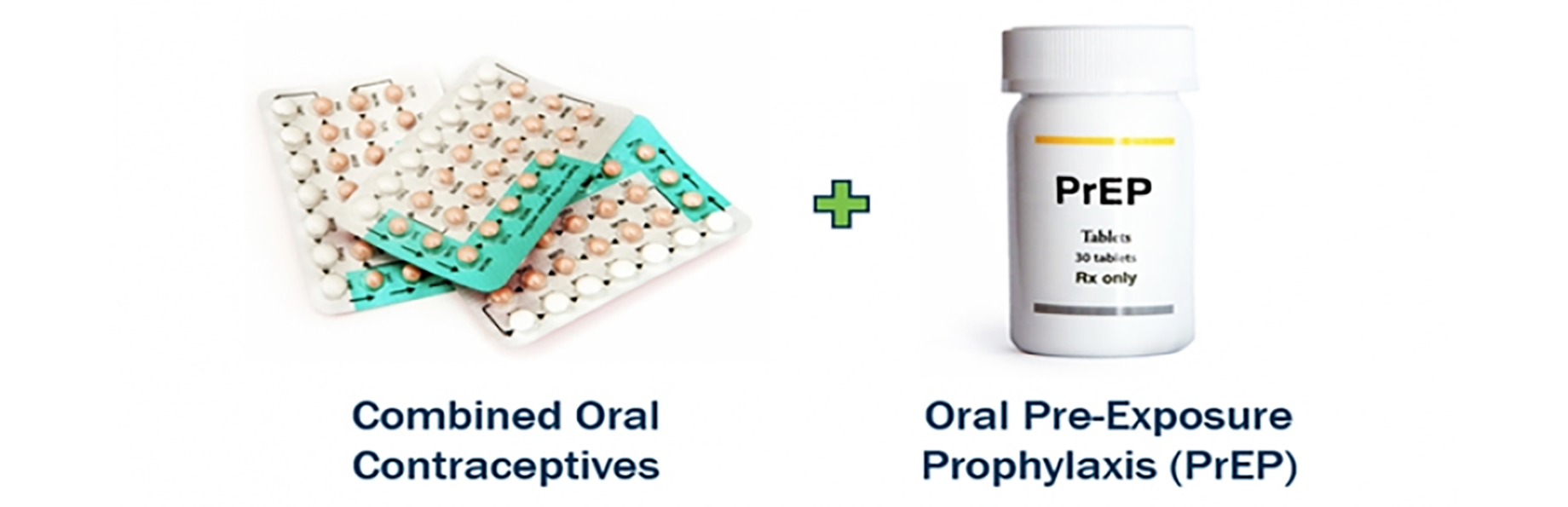
Dual Prevention Pill for the Prevention of HIV and Unintended Pregnancy
Evidence shows more women would use an HIV prevention method if it was combined with a contraceptive. Together with Medicines360, we’re developing a dual prevention pill that combines oral contraceptives pills with PrEP to meet this need.
Novel Intravaginal Ring as a Nonhormonal Contraceptive
Scientists are developing a nonhormonal, user-controlled vaginal ring with the potential to provide contraception while protecting against STIs and bacterial vaginosis. Research and development is funded by an $11 million P50 Clinical Research Center Grant from the Eunice Kennedy Shriver National Institute for Child Health and Development (NICHD) of the National Institute of Health (NIH). Queen’s University Belfast and Weill Cornell Medical College will partner with the Council on this grant over the next five years.
The Center for Biomedical Research welcomes your collaboration. We partner with universities, government institutions, other nonprofit research organizations, service delivery organizations, contract research organizations, and pharmaceutical companies to develop, manufacture, and conduct clinical trial testing.
For more information, please contact: Rebecca Brodsky, Project Manager, rbrodsky@popcouncil.org
To support our work, contact: philanthropy@popcouncil.org