Comprehensive sexual and reproductive healthcare supports women on whether, when, and how many children they want to have throughout their 30+ year reproductive life span. Unfortunately, global gaps remain, and almost half of all pregnancies are unintended. Side effects and health concerns with existing methods are often the most-cited reason for non-use or discontinued use of contraception. Therefore, new methods of contraception that could better meet women’s needs throughout their lives could help alleviate rates of unintended pregnancy.
At the Population Council’s Center for Biomedical Research, we use an end-to-end approach to take SRH products from the lab to the hands of users. This approach has led to the successful development of eight approved technologies for contraception and HIV prevention, in addition to other scientific learnings that have supported advances in the field. Our current portfolio includes many novel technologies to better meet SRH needs, including next generation contraception for men and women, novel HIV prevention methods, and multipurpose prevention technologies (MPTs) to protect against multiple indications simultaneously.
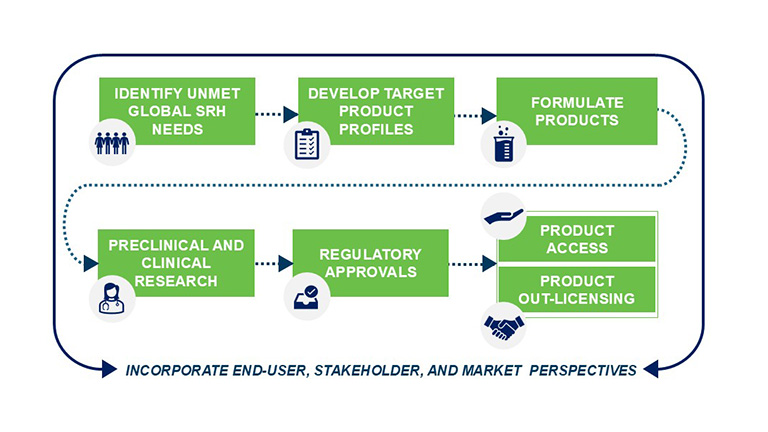
We begin the product development process by identifying unmet, global sexual and reproductive health needs through epidemiologic trends, acceptability data, and global health priorities. Inputs from in-country partners help us determine which products are most needed. We consult global and local partners and government stakeholders to integrate broader socio-ecological considerations, including societal norms, values, institutions, and environmental factors.
After a target or need is identified, we develop target product profiles that define our minimally acceptable versus ideal product attributes. These attributes help us steer product formulation and preclinical and clinical development. The targets are set based on learnings regarding safety, efficacy, stability, and cost from other products and the target market; feedback from stakeholders; and extensive socio-behavioral research conducted among women and girls. Defining these targets early on ensures the products we are developing meet the needs and desires of women and girls.
Our laboratory scientists work with active pharmaceutical ingredients to formulate products based in vaginal rings, topical gels, oral pills, fast-dissolving inserts, and other novel delivery systems.
We then begin preclinical research, meaning research that is done before it is tested on humans, to rigorously test products for safety and efficacy. Once preclinical models confirm the product is safe and effective, we collaborate with academic and commercial partners to conduct clinical research to test the products in humans. We ensure research transparency by publishing our results in in high-impact journals. Alongside this safety and efficacy research, we explore the acceptability of our products among potential end-users to ensure the products are appealing and acceptable.
To secure regulatory approvals, we collaborate with a range of global, national, and local stakeholders to chart the most efficient regulatory pathways to product approvals with stringent regulatory authorities (such as the United States Food and Drug Administration and the European Medicines Agency) and other national authorities.
In parallel with the product development and regulatory processes, we develop product access strategies. This can involve working with local and global stakeholders, such as the World Health Organization, to secure prequalification. Additionally, we engage with local government, research, and advocacy partners to develop clinical guidance, educational tools, and streamlined supply chain processes.
We employ a flexible product out-licensing approach in which we seek capable partners to manufacture and distribute our products. We negotiate pricing terms to support access in low- and middle-income countries and public sector markets. We advocate for product inclusion in country planning and budgeting, and policy advocacy for procurement. We engage various stakeholders, including ministries of health, healthcare providers, end-users, community leaders, and advocacy groups early and often to build excitement and demand for new products. As demand grows, new products can be scaled up at competitive prices.
By utilizing this end-to-end product development approach, we have the capability to advance a product from the lab to the hands of users. This approach is guided by principles of access, cost, user desires and preferences, and ultimate global health impact. As a result of this comprehensive approach, 170 million women today are using technologies developed by the Population Council or based on Council technology.
