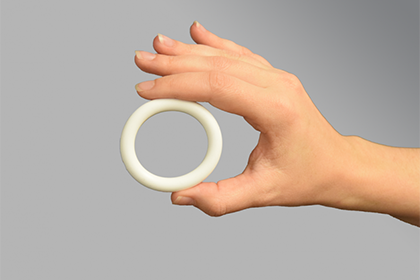
Identifying gaps within current contraceptive options is essential to expanding access for all individuals. Currently, several contraceptives provide limited options for individuals with estrogen sensitivities, those at higher risk for venous thromboembolism (VTE), or postpartum and breastfeeding women. It’s essential that individuals of reproductive age in need of contraception have a variety of contraceptive methods to choose from in order to select the one best suited for their individual needs. Often times, women opt for more discreet methods of birth control as a way to preserve private autonomous decisions about their health. One such method is the intravaginal ring, a device that has risen in popularity over the years due to its discreet and ease of use.
Researchers at the Population Council’s Center for Biomedical Research are developing a 3- and 6-month intravaginal contraceptive ring using Nestorone®, a progestin-only compound, designed for all women of reproductive age in need of contraception. This woman-controlled device does not require refrigeration or insertion by a skilled health care provider, thus providing greater autonomy to users of this method who can insert and remove the device by themselves. Similar to other vaginal rings, there is expected to be minimal issues for the user related to expulsions, or the ejection of the ring. The Council will soon file an investigational new drug application and conduct a phase 2 dose-finding study to select the optimal ring to advance.
With no BMI restrictions, the Nestorone-only intravaginal ring will have several advantages to its counterparts serving as a great option for a wide audience of individuals at risk of pregnancy, including but not limited to, those who have estrogen sensitivities, those who are at higher risk of VTE, and postpartum breastfeeding mothers. Postpartum women have limited options for contraception as they are often advised against using hormonal methods containing ethinyl estradiol due to ethinyl estradiol’s effect on milk production or composition. Thus, using a method with Nestorone®, a progestin that is not orally active, is show to be exceptionally safe for breastfeeding women and their infants. Users of the Nestorone-only intravaginal ring are expected to have an improved-side effect profile including minimized effects on bone density and a rapid return to fertility.