Every day, more than 1 million people contract sexually transmitted infections (STIs), the majority of which are asymptomatic. STIs can have a direct impact on sexual and reproductive health through stigmatization, infertility, cancers, and pregnancy complications and can increase the risk of contracting HIV.
Additionally, bacterial vaginosis (BV) is the most common vaginal infection among women of reproductive age and is associated with adverse health outcomes. Global prevalence of BV is high, ranging from 23% to 29% across regions. Women with BV are at increased risk of acquiring STIs and have an increased risk for pregnancy complications.
Given the growing burden of sexually transmitted infections, many women desire a multipurpose prevention technology (MPT) for protection against pregnancy, HIV, and other STIs. The MPT development space is dominated by hormonal methods. Despite their attractiveness, hormonal candidates also have attributes that could limit their acceptability, including safety risks, side effects, irregular bleeding, and contraindications. These attributes can be unsuitable for some women seeking an MPT, especially those who are breastfeeding, have infrequent sex, or are unable or unwilling to use hormonal contraception.
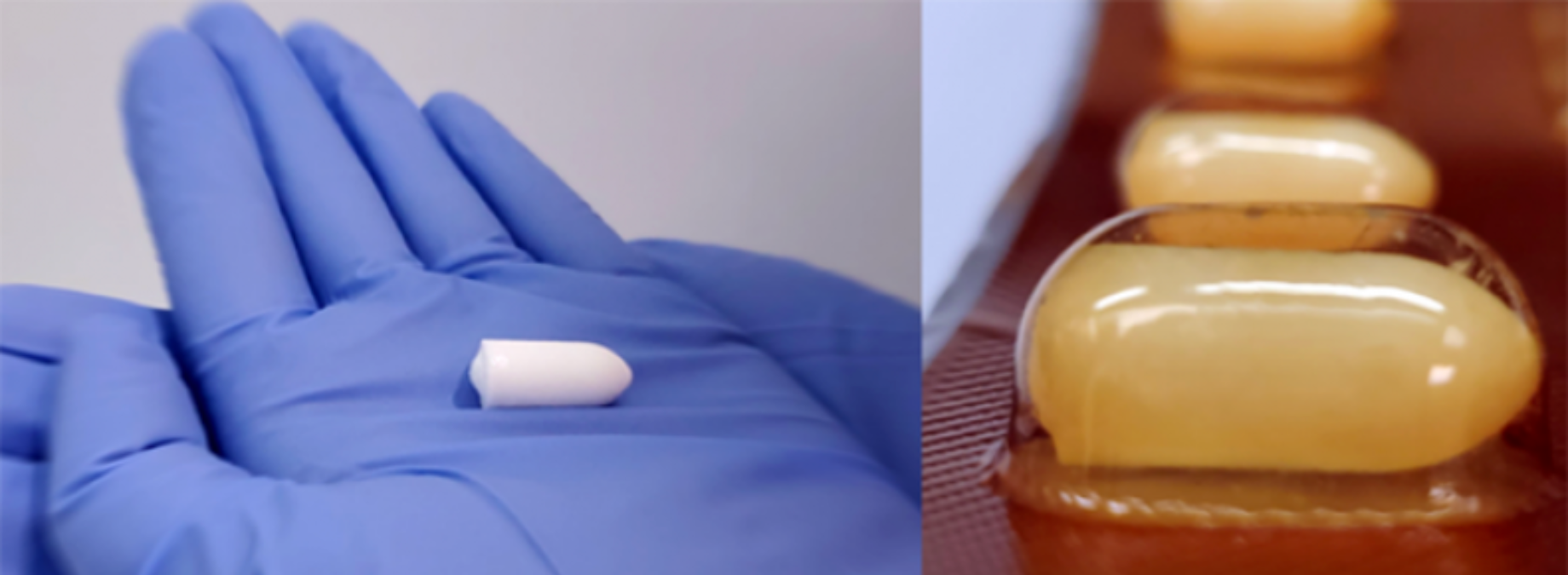
Q-Griffithsin is a non-antiretroviral (non-ARV) lectin with potent anti-HIV activity. The Population Council is formulating these active ingredients, combined with excipients, into an FDI for on-demand use prior to sex. An FDI is a small solid topical insert that comes in a blister-pack. When inserted into the vagina, the FDI dissolves into a viscous gel delivering the active agents throughout the vaginal lumen.
FDIs are inexpensive, discreet, portable products that have tested well in acceptability surveys and are currently marketed for other indications. FDIs are an emerging on-demand drug delivery system with which the Population Council has significant experience and compelling in vivo data. The Q-Griffithsin contraceptive MPT FDI would be designed to be self-inserted (with fingers) at or around the time of sex to prevent pregnancy, sexually transmitted infections, and bacterial vaginosis.
The Population Council, in collaboration with our partners, is currently evaluating the FDI in a series of in vitro assays for anti-sperm and anti-STI activity and stability, and in vivo for safety and efficacy. After these evaluations, the product will be manufactured under good manufacturing practices and advance to toxicology studies to enable an Investigational New Drug application, and contraceptive efficacy studies. The final deliverable from this work will be a contraceptive MPT FDI that will be ready to test in a Phase 1 clinical trial.
If successful, this will be the first on-demand, nonhormonal, non-ARV contraceptive MPT for the prevention of pregnancy, HIV/STI infection, and bacterial vaginosis. To our knowledge, there is no other MPT on the market, or in development, that includes such a wide range of activities against various STIs in addition to exerting a contraceptive effect. Unlike most HIV-prevention drugs in development, Q-Griffithsin is a non-antiretroviral inhibitor of HIV, which could help reduce the emergence of drug-resistant HIV. Because it is locally administered and not systemically absorbed, its safety and effectiveness should not be affected by variation in body mass index.
Our FDI has the potential to fill the unmet need of users who desire a nonhormonal contraceptive MPT for protection against pregnancy, HIV/STIs, and bacterial vaginosis. This user-controlled FDI would be discreet, easy to self-administer, low-cost, compactly packaged, and stable at room temperature across most climatic zones.




